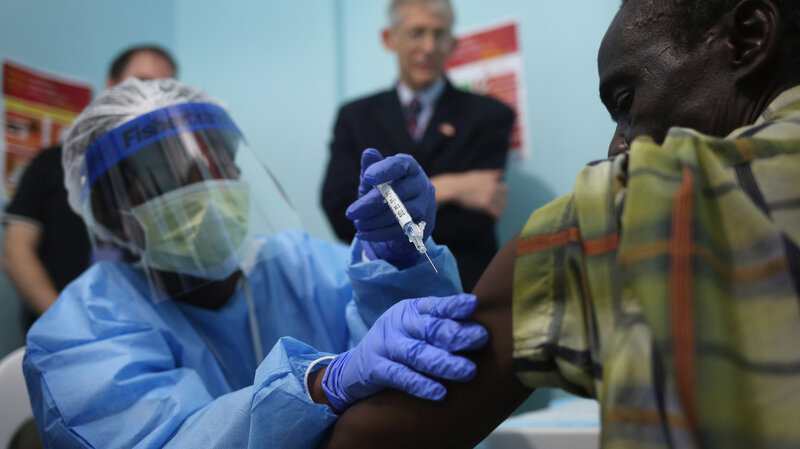
On Monday, the first 12 volunteers received an experimental Ebola vaccine in Liberia, launching vaccine trials
there. Over the next year or so, scientists hope to inject 27,000
volunteers. The goal is to test two different shots that could protect
people from the deadly disease.
But the number of Ebola cases
is steadily declining across West Africa. And that good news is
hampering drug and vaccine development, especially in Liberia. The
country recorded only four Ebola cases last week, the World Health
Organization said.
"We're in a bit of a strange situation," says WHO's Martin Friede, who heads the group coordinating Ebola drug development.
"On
the one hand, the number of Ebola cases has really dropped
dramatically. ... This is excellent for the countries involved," Friede
says. "However, from a drug research and development perspective, this
is not so good."
The reason is quite simple.
"It's very difficult conducting clinical trials when there are very few actual patients," Friede says.
One of the two drugmakers gearing up for clinical trials has given up. North Carolina-based Chimerix announced Friday that it would halt a planned test of its drug, brincidofover, in Liberia.
Countdown To Zero: Guinea's Campaign To Conquer Ebola In 60 Days
The only other drug that has been part of an active trial is a Japanese flu medication called favipiravir. It's being tested in Guinea, and the trial has already recruited enough patients. "So
at least there will be one trial where they will probably have had
adequate numbers for [researchers] to make some estimate of efficacy, or
at least of safety," Friede says.
The story with preventive
vaccines is not quite so dire. The trials starting this week aim to
inject 9,000 people with one vaccine, another 9,000 with a second
vaccine, and another 9,000 with a placebo injection, over the next 12 to
15 months.
Vaccine researchers need healthy volunteers, not sick people. If
nobody gets exposed to Ebola after getting the shot, doctors won't be
able to tell for sure if the vaccine is effective. But there's value in
these experiments, even if the epidemic fades away.
Where Could Ebola Strike Next? Scientists Hunt Virus In Asia
"There would still be a considerable amount of important data that one can get from a vaccine trial," says Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases.
Blood
tests can measure the immune system response in people. If that
response closely resembles the immune response in monkeys — where the
vaccine has been shown to be protective — it's possible that a vaccine
could be approved on that basis alone, Fauci says.
Another
possibility is to expand the vaccine trials into Sierra Leone and
Guinea, which reported 65 and 30 cases last week, respectively.
"That's not up to us," Fauci says. "We respect the countries' need to be able to make those decisions on their own."
Picking
up and moving to another country is much harder for drug trials than
for vaccine trials. After a country gives its approval, WHO's Friede
says, researchers still need to make sure that care meets a minimum
standard where the drug is tested. There also need to be enough nurses
and doctors on hand, and they need to be trained in the conduct of
clinical trials.
"So this is not at all easy," Friede says.
At this point, Friede is starting to talk about making sure the drug tests are ready to go for the next
Ebola epidemic — although health authorities still need to remain
vigilant, he says, to make sure this one is in fact coming to an end.
No comments:
Post a Comment